14 December 2007
The 2nd Forum of the European Qualified Person Association a Complete Success
From 22 - 23 November 2007, the 2nd QP Forum of the European QP Association
took place in Berlin, Germany. On the 21st, a Pre-Conference Workshop was
held for the recently founded IMP QP Subgroup of the QP Association.
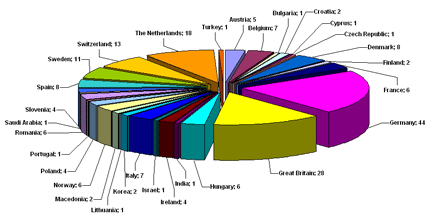 Dr Bernd Renger, Chairman of the EQPA welcomed more than 200 delegates,
representing almost 30 countries. Qualified Persons, executives from the
pharmaceutical industry and representatives from various national
authorities informed themselves about the latest developments. Dr Bernd Renger, Chairman of the EQPA welcomed more than 200 delegates,
representing almost 30 countries. Qualified Persons, executives from the
pharmaceutical industry and representatives from various national
authorities informed themselves about the latest developments.
Bernd opened the Forum with an overview about the activities of the EQPA
which currently has close to 900 members and associate members. One
important step in the young history of the Association was taken just a few
weeks before the Forum. Dr Bernd Renger and Richard Bonner represented the
EQPA at the EMEA GMDP IWP Interest Parties Meeting in London. Beforehand, a
survey was conducted to identify the expectations of the EQPA members. Based
on the information given by the members, the Board members were able to
provide substantial and comprehensive feedback on EMEA's Position Paper on
QP Discretion.
The first speaker, EMEA's Piotr Krauze, gave a detailed overview about the
role of EMEA and the QP within the European legislative framework. Piotr
encouraged the QPs to keep abreast of new quality and GMP developments. The
QP Association should facilitate the continuous learning process and
contribute to proposed legislation and guidance. EMEA also asks the QP
Association to be prepared to provide upon request from the EMEA information
on specific topics, practical examples and experiences and to point out
operational problems within legislative framework.
Other speakers from the EQPA, the pharmaceutical industry and national
regulatory authorities shared their views on roles and responsibilities of
Qualified Persons. Release requirements for APIs, Biotech and Blood Products
were presented and the various aspects what personal and technical skills a
QP needs to have were elaborated.
 The European Qualified Person Association also invited representatives from
other interest groups for the 2007 Forum. John O'Sullivan who represented
EFPIA pointed out the strong need to update EU GMPs to clarify the QP
responsibilities. He also suggested speaking of accountabilities, not of
responsibilities. The QP should also be allowed to delegate duties (not
responsibilities) to non-QPs. Those duties should not be further expanded in
future updates of the EU GMPs. It should be beneficial for the QPs if the
EMEA Reflection Paper on QP Discretion was incorporated with the feedback
given into EU GMPs. Consistency across the Member States would be highly
appreciated. The European Qualified Person Association also invited representatives from
other interest groups for the 2007 Forum. John O'Sullivan who represented
EFPIA pointed out the strong need to update EU GMPs to clarify the QP
responsibilities. He also suggested speaking of accountabilities, not of
responsibilities. The QP should also be allowed to delegate duties (not
responsibilities) to non-QPs. Those duties should not be further expanded in
future updates of the EU GMPs. It should be beneficial for the QPs if the
EMEA Reflection Paper on QP Discretion was incorporated with the feedback
given into EU GMPs. Consistency across the Member States would be highly
appreciated.
Graça Mata talked about the expectations of the Active Pharmaceutical
Ingredients Committee (APIC), which are in line with those of EFPIA and
EQPA. An alignment of the responsibilities and duties of QP and the
enforcement of the respective directives across the EU should be the overall
goal.
A set of parallel sessions gave the delegates the possibility to work on
various case studies. Possible problems and scenarios were thoroughly
discussed. The focus of these interactive sessions was on Batch Release,
import and IMP requirements.
 The QP Forum was completed by a highly appreciated social event on Thursday
evening. After a guided city tour through Berlin, the participants had the
possibility to continue their discussions and share their experiences with
their colleagues in a relaxed atmosphere in the Wasserwerk, an event hotspot
in Berlin. The QP Forum was completed by a highly appreciated social event on Thursday
evening. After a guided city tour through Berlin, the participants had the
possibility to continue their discussions and share their experiences with
their colleagues in a relaxed atmosphere in the Wasserwerk, an event hotspot
in Berlin.
Besides the extensive programme, the aim of the 2nd Forum was also to
present a platform for sharing experiences and discussing the various
aspects of the QP's daily work. The Advisory Board encouraged the members to
further support the Association in their current and future activities and
also asked for ideas and active participation in the next QP Forum which
will be held from 3 - 5 December 2008 in Munich, Germany.
Author:
Wolfgang Schmitt
On behalf of the European QP Association
<< Back to overview
|



